The Low Down on Manufacture Of Caustic Soda By Castner Kellner Cell Revealed |
Although demand for caustic soda had been sturdy in the US, the autumn in consumption in the pulp and paper, alumina and manufacturing industries because of the economic crisis has affected demand. The market for US caustic soda looks grim for 2009 and it is extremely probably that North American chlor-alkali producers will curtail manufacturing. Between 2003 and 2008, the net enhance in demand has averaged 2m tonnes/12 months while capacity has grown at a median of 2.5m dry tonnes/year, notes CMAI.
Consider the health of the myocardium earlier than choosing which sort of bronchodilator must be administered. Cardiac sensitizing brokers could also be applicable; nevertheless, using cardiac sensitizing brokers after publicity to certain chemical compounds may pose enhanced risk of cardiac arrhythmias (especially sodium hydroxide prices in the elderly). Sodium hydroxide poisoning isn't known to pose further threat throughout using bronchial or cardiac sensitizing agents. The severity of sodium hydroxide burns may not be readily obvious until 24 to forty eight hours after exposure.
Caustic Soda Uses
However, laboratory testing is useful for monitoring the affected person and evaluating issues. Routine laboratory research for all uncovered patients embody CBC, glucose, and electrolyte determinations.
However, the lye, used to make soap, is no longer within the cleaning soap in the form of an ingredient in the completed client product. Careful storage is needed when handling sodium hydroxide to be used, particularly bulk volumes.
Inhalation of sodium hydroxide is immediately irritating to the respiratory tract. Swelling or spasms of the larynx resulting in upper-airway obstruction and asphyxia can occur after high-dose inhalation. Inflammation of the lungs and an accumulation of fluid within the lungs may occur. Sodium hydroxide is used to fabricate soaps, rayon, paper, explosives, dyestuffs, and petroleum products.
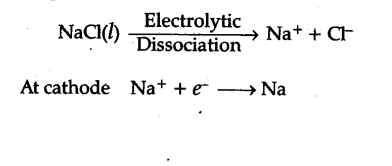
How will you prepare caustic soda from brine?
- It is mostly prepared by electrolysis of sodium chloride in Castner-kellner cell.
Extensive pores and skin burns or gastrointestinal-tract harm from ingestion might compromise fluid steadiness, inflicting shock; early medical look could not predict this event. Stridor, vomiting, painful swallowing, drooling, and stomach pain are early signs of sodium hydro xide ingestion. Sodium hydroxide dissociates within the body and wouldn't attain the reproductive organs in an unchanged state.
Depending on the aim, you might be able to substitute a chemically similar strong base, potassium hydroxide (KOH). This is a chemical you can, in case sodium hydroxide exporters you are extremely dedicated, make yourself by soaking wooden ashes in water. To do that, soak a large quantity of ashes in a small amount of water.
The Castner Kellner course of is a technique of electrolysis of sodium chloride answer to supply alkali hydroxide. It uses electrolyte of NaCl solution graphite anode and mercury cathode. In other cell sodium hydroxide is an electrolyte, Hg anode is used as an intermediate between two cells and an iron cathode. A resolution Iran caustic soda of sodium hydroxide in water was traditionally used as the most common paint stripper on picket objects.
Corrosive injury to the mouth, throat, esophagus, and abdomen is extremely speedy and may end in perforation, hemorrhage, and narrowing of the gastrointestinal tract. Ingestion may lead to perforation of the gastrointestinal tract and shock. Sodium hydroxide may cause hydrolysis of proteins, and therefore could cause burns within the eyes which may lead to everlasting eye harm. Ingestion of sodium hydroxide could cause extreme corrosive injury to the lips, tongue, oral mucosa, esophagus, and stomach.
At some point it'll crystallize out and - as Kevplumb says - it could possibly even turn stable; the water becomes part of the crystal construction. I cleared the blockage within the outside drain last time with caustic soda granules, mixed with water - nonetheless, the drain is very awkward to get to. lye for soap making to be best Caustic Soda is activated by scorching water, so don't just put it on a rinse cycle. https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-hydroxide would not advocate anybody to make use of it until they are absolutely sure that they know the right method to deal with it - always add the powder to the water, not the opposite way spherical.
| Комментировать | « Пред. запись — К дневнику — След. запись » | Страницы: [1] [Новые] |






