What Is Radon Testing Things To Know Before You Buy |
EPA life time safety standards for carcinogens are developed based on a 1 in 100,000 threat of death. A lot of researchers concur that the risk of fatality for radon at 4 p Ci/L is around 1 in 100. At the 4 p Ci/L EPA radon action guideline degree, lugs approximately 1000 times the threat of death as any kind of other EPA carcinogen.
It is crucial to keep in mind that the activity degree is not a secure level, as there are no "secure" levels of radon gas. Radon is a cancer-causing radioactive gas. You can not see, scent or preference, yet it may be a trouble in your. The Specialist General has advised that is the second leading root cause of in the United States today.
Some clinical of exposure indicate that children might be a lot more conscious. This might be due to their greater respiration rate as well as their swiftly splitting cells, which might be much more at risk to radiation damages. Radon is an anemic chemically-unreactive inert gas. The atomic distance is 1.34 angstroms as well as it is charcoal radon test the heaviest recognized gas-- radon is nine times denser than.
The What Is Radon Testing Ideas
Radon is likewise rather soluble in and also natural solvents. Although response with other substances is relatively uncommon, it is not completely inert and types stable molecules with extremely electronegative materials. is taken into consideration a worthy gas that happens in a number of isotopic kinds. Only 2 are located in considerable concentrations in the human setting: -222, as well as -220.
-220 https://tapas.io/radon1 is developed in the decay chain of thorium-232. -222 degenerations in a sequence of radionuclides called degeneration items, children, or kids. It is -222 that a lot of easily happens in the environment. Atmospheric releases of -222 results in the development of decay items that are radioisotopes of hefty steels (polonium, lead, bismuth) as well as quickly affix to various other air-borne materials such as dust and other materials facilitating inhalation.
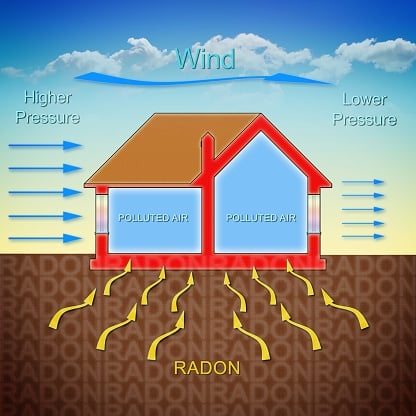
- The Best Guide To How To Get Rid Of Radon
- Unknown Facts About What Is Radon Testing
- The 3-Minute Rule for How To Get Rid Of Radon
- The Main Principles Of What Is Radon Testing
is a normally happening contaminated gas as well as originates from the natural break down (radioactive degeneration) of uranium. It is normally found in igneous rock and also dirt, but sometimes, well might likewise be a resource of. EXPOSURE: The primary paths of prospective human direct exposure to are breathing and consumption.
Fascination About What Is Radon Testing
Although high focus of in groundwater might contribute to direct exposure through consumption, the inhalation of launched from is normally more crucial. IN THE WORKPLACE: In comparison with levels in outdoor, humans in restricted rooms, especially in below ground job areas such as mines as well as structures, are subjected to elevated concentrations of and also its degeneration items.
The typical concentrations in are generally much less than the average focus in below ground ore mines. Workers are exposed to in a number of professions. In countries for which information were readily available, concentrations of degeneration items in below ground mines are now commonly much less than 1000 Bq/m 3 EEC Rn (approx. 28 p Ci/L).
Other underground workers as well as particular mineral processing employees might likewise be subjected to substantial degrees. Evaluating is the only means to recognize your radon level. There are no immediate symptoms that will certainly notify you to the presence of radon. It normally takes years of exposure before any troubles surface. The US EPA, Surgeon General, American Association, American Medical Organization, and also National Security Council suggest examining your residence for radon due to the fact that screening is the only means to recognize your radon degree.
Our How To Get Rid Of Radon PDFs
The US EPA approximates that as several as 8 million throughout the country have elevated levels of radon. Current state surveys show that 1 in 5 has elevated radon degrees. It's best to count on a professional-- specifically when handling a health hazard. In fact, several UNITED STATE states require radon experts to be certified and accredited in their field.
chemical component with atomic number 86 Chemical element with atomic number 86Radon, 86Rn Radon Pronunciation(RAY-don) Appearancecolorless gasMass numberRadon in the routine table Atomic number (Z) 86Teamteam 18 (noble gases)Periodperiod 6 Blockp-block Component category Noble gasElectron setup [Xe] 4f 14 5d 10 sixes 2 6p 6Electrons per covering 2, 8, 18, 32, 18, 8Physical propertiesPhase at STPgasMelting point202 K( − 71 ° C, − 96 ° F) Boiling factor211.5 K( − 61.7 ° C, − 79.1 ° F) Thickness (at STP) 9.73 g/Lwhen fluid (at b.p.) 4.4 g/cm 3 Vital point377 K, 6.28 MPa Warmth of blend3.247 k J/mol Heat of evaporation18.10 k J/mol Molar warm capacity5R/ 2 = 20.786 J/( mol · K ) Vapor pressure P (Pa) 1 10 100 1 k 10 k 100 k at T (K) 110 121 134 152 176 211 Atomic residential or commercial properties Oxidation states 0,+2, +6 Electronegativity Pauling scale: 2.2 Ionizationenergies Covalent radius 150 pm Van The original source der Waals radius 220 pm Spectral lines of radon Various other buildings All-natural event from degeneration Crystal framework face-centered cubic (fcc) Thermal conductivity 3.61 × 10 − 3 W/( m· K) Magnetic getting non-magnetic CAS Number 10043-92-2 Background Discovery Ernest Rutherford and also Robert B.
| Комментировать | « Пред. запись — К дневнику — След. запись » | Страницы: [1] [Новые] |






