Just how to Care for Your Caustic Soda Reaction With Chlorine Gas |
When chlorine is passed via hot concentrated caustic soda answer, the oxidation number of chlorine in one of the products increases from:
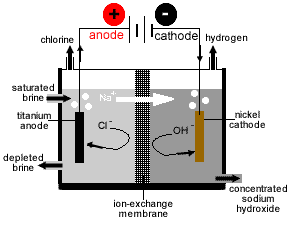
4 HCl + O2 → 2 Cl2 + 2 H2OThis response is accomplished with using copper(II) chloride (CuCl2) as a catalyst and is performed at excessive temperature (about 400 °C). Due to the extraordinarily corrosive reaction combination, industrial use of this methodology is tough and a number caustic soda flakes of other pilot trials failed in the past. Recently Sumitomo patented a catalyst for the Deacon course of utilizing ruthenium(IV) oxide (RuO2). Chlorine and hydrogen gases are shaped when concentrated aqueous NaCl is electrolyzed to supply NaOH. To stop the response of chlorine and NaOH, a membrane is placed between cathode and anode.
It's additionally much less dependable since chlorine bleach often decays on the shelf to lower concentrations. Another earlier process to produce chlorine was to warmth brine with acid and manganese dioxide.
Can breathing chlorine make you sick?
https://www.brenntag.com/en-us/products/caustic-soda-guide/index.jsp and Cl2 reaction | NaOH + Cl2 = NaCl + NaOCl + H2O. Cold dilute sodium hydroxide and chlorine gas reaction is disproportionation. Chlorine gas is oxidized and reduced to OCl- ion and Cl- ion respectively. As products sodium chloride, sodium chlorate(i) and water are given.
In animal fashions, exposure to 200 ppm leads to intensive bronchial constriction. Exposure to levels of 800 ppm proves deadly to half of all exposed animals while concentrations of 2000 ppm lead to quick respiratory arrest. Experimental research utilizing a low focus of chlorine fuel /ishtarcompany.com/caustic-soda-flakes/">https://ishtarcompany.com/caustic-soda-flakes/ ">where to buy caustic soda present that the upper airway absorbs the vast majority of inhaled chlorine. Animal models suggest that chlorine fuel absorbed in the decrease respiratory tract causes much larger toxicity than similar quantities within the higher airway.
The simplest method to make chlorine gasoline is to mix bleach with hydrochloric acid. While this does work it would what is caustic soda not produce as a lot chlorine gas as the opposite strategies.
- Chlorine gas is also essentially the most frequent explanation for main poisonous release incidents internationally.
- The caustic exiting the cell line must be monitored for power, to take care of secure concentrations.
Most exposures to chlorine gasoline will present with a clear historical past of publicity obtained either from the patient themselves or first responders arriving from the scene. From prior exposures at chlorinated swimming pools, most sufferers will acknowledge the distinct odor of chlorine gas. Rarely a affected person in respiratory distress and altered psychological status could also be discovered after the whole dispersal of chlorine fuel making the history unclear. Salivation, lacrimation, rhinorrhea, and bronchospasm can happen in cholinergic toxicity as well as chlorine gasoline exposure. Symptoms of chlorine gas publicity are normally various depending on the kind of exposure.
What happens when chlorine is passed?
When chlorine is passed over dry slaked lime, bleaching powder will form. equation ---: Ca(OH)2 + Cl2 ---> CaOCl2 + H2O. properties --: it is used as an oxidising agent in many chemical industries. it is used for bleaching cotton , linen in textile industry.
Chlorine gas has been used as an agent of war as just lately as 2007 in Iraq. Because of its strong odor, chlorine gasoline can be detected easily. Symptoms of chlorine fuel exposure embrace burning of the conjunctiva, throat, and the bronchial tree. Higher concentrations can produce bronchospasm, lower sodium hydroxide sellers pulmonary damage, and delayed pulmonary edema. Toxicity to chlorine fuel is determined by the dose and duration of publicity.
For acute exposure at low ranges (lower than 5 ppm), sufferers can have lacrimation, nose and throat irritation, and excess salivation. Chronic publicity to chlorine gas can result in chest ache, cough, sore throat, and hemoptysis. When exposed to low focus chlorine gas (up to 2 ppm) mucous membrane irritation results. Higher focus exposures between 9 ppm and 50 ppm could result in chemical pneumonitis and bronchiolitis obliterans.
One of the worst was a 2007 collision of a railroad tanker carrying chlorine with another prepare causing rupture of the tank and launch of 90 tons of chlorine gasoline into the encircling caustic soda sellers area. Exposure to chlorine gas at the web site of the accident resulted in 9 fatalities and 520 visits to native emergency departments in Graniteville, South Carolina.
| Комментировать | « Пред. запись — К дневнику — След. запись » | Страницы: [1] [Новые] |






