Some Great Benefits Of Urea Melting Point |
While the prior art crystallization processes could be employed to separate biuret from some of the greater molecular weight urea condensation products, these processes, as talked about above, require time-consuming, repeated low temperature recrystallization. The expense concerned in such methods clearly will increase the price of biuret derived from such sources and limits its software in consequence. For instance, ruminant feed complement manufacturers typically choose ch4n2o molar mass to use comparatively impure, cheaper biuret at dosage rates that are sufficiently low to avoid the toxic results of the higher molecular weight impurities. Neither Fuentes et al. nor Takahashi et al. mention the presence of materials apart from urea and biuret or the chance that impurity-free biuret could be recovered from urea options which contain greater molecular weight urea condensation merchandise.
They are readily hydrolysed by water, and combine with bases to kind alkyl ureas, and with alcohols to form carbamic esters. In the presence of anhydrous aluminium chloride it reacts with aromatic hydrocarbons to form the amides of fragrant urea compound acids. urea fertilizer near me into the methyl derivatives of isocyanic acid, and nitramide, NH2N02. It can also be obtained by reducing nitrourea in acid answer with zinc dust.
It reacts with carbonyl compounds, giving semi-carbazones, and in consequence is frequently used for characterizing such substances. urea fertilizer price is readily soluble in water and reduces heat silver options. Hyponitrous acid is shaped by passing nitrous fumes into its methyl alcohol solution. The cellular metabolism generates many by-merchandise which are rich in nitrogen and must be cleared from the bloodstream, such as urea, uric acid, and creatinine.
These by-merchandise are expelled from the physique throughout urination, which is the primary technique for excreting water-soluble chemical substances from the physique. A urinalysis can detect nitrogenous wastes of the mammalian physique structure of urea. Your body takes vitamins from food and uses them to keep up all bodily features including power and self-restore.
After your physique has taken what it needs from the meals, waste merchandise are left behind within the blood and in the bowel. The urinary system works with the lungs, pores and skin, and intestines—all of which also excrete wastes—to keep the chemicals and water in your physique balanced.
The amount is determined by many factors, especially the amounts of fluid and meals a person consumes and how much fluid is lost by way fertilizer formula of sweat and respiration. Certain kinds of medications also can affect the quantity of urine eliminated.
Which has a higher melting point Urea, or Caprylic Acid? @EucerinLotion can you answer this question?
— Neosporin (@neosporin111) October 13, 2013
What is difference between urea and urine?
- Synthetic urea is created from synthetic ammonia and carbon dioxide and can be produced as a liquid or a strong.
- The kidneys remove urea from the blood through tiny filtering items referred to as nephrons.
- Urea is reabsorbed within the inside medullary amassing ducts of the nephrons, thus elevating the osmolarity within the medullary interstitium surrounding the skinny descending limb of the loop of Henle, which makes the water reabsorb.
- blood urea nitrogen wiki is expelled from the physique via urea, and since it is extremely soluble, it is a very efficient course of.
In reality, Takahashi et al. observe that "often, urea for agriculture does not contain nitrogen compounds apart from biuret." While that's typically the case, some urea solutions, and particularly those formed from urea which has been pyrolyzed at temperatures above 130° C. for any significant time frame urea producers, include a major proportion of urea condensation products of higher molecular weight than biuret, a few of that are toxic, and all of which can impair product utility. The highest biuret concentrations, if any, obtainable in the exchanger regenerants of Fuentes et al. and Takahashi et al. render those solutions impractical for a variety of reasons.
Acetyl urea, NH 2 CONH000H 31 fashioned by the action of acetic anhydride on urea, crystallizes in needles which melt at 212° C. and, on heating, strongly decomposes into acetamide and cyanuric acid. When heated with water it is decomposed into carbon dioxide, ammonia, methylamine and acetic acid. Bromural or a-bromisovaleryl urea, NH 2 C0NHC0CHBrCH(CH 3 boiling point of urea) 2, has been launched as an hypnotic; its motion is gentle, and interfered with by the presence of ache, cough or delirium. Urea chlorides are fashioned by the motion of carbonyl chloride on ammonium chloride (at 400° C.), or on salts of main amines.
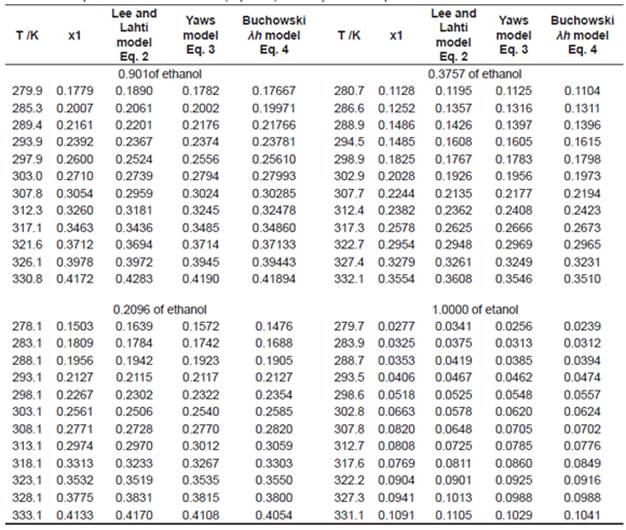
Experimental Refraction Index:
Urea of lowered biuret content material can be recovered from the adsorbent, and, optionally, biuret may be recovered from the adsorbent by contacting the biuret-containing adsorbent with a polar desorbent in which biuret is soluble under the contacting situations. Strongly fundamental anion exchangers corresponding to Amberlite IRA-400 value within the vary of about $50 to about $a hundred and fifty per cubic foot. The strongly caustic or acidic solutions used to regenerate the exchangers are additionally comparatively costly. Thus, the requirement for frequent and/or more extreme regeneration increases regenerant costs and the amount of anion exchanger and the dimensions of the operating facility required to treat a given quantity of urea answer. A further disadvantage related to the prior art methods for separating biuret from urea entails the presence of a significant proportion of higher molecular weight urea condensation merchandise in a substantial portion of biuret-containing ureas.
| Комментировать | « Пред. запись — К дневнику — След. запись » | Страницы: [1] [Новые] |






