Reasons Why Caustic Soda Manufacture Gets Bad Opinions |
A hypochlorite incident is a direct reaction of severe ache, followed by edema, haematoma, and ecchymosis as a consequence of the solution escaping the confines of the tooth and getting into the periapical space. This could also be brought on by binding or extreme stress on the irrigant syringe, or it might occur if the tooth has an unusually massive apical foramen. Liquids containing sodium hypochlorite as the primary caustic soda cost active component are additionally used for household cleaning and disinfection, for instance toilet cleaners. Some cleaners are formulated to be viscous so as to not drain quickly from vertical surfaces, such as the inside of a bathroom bowl.
When the patient reported that the ingested quantity was a lot lower than 22 g, we outlined this quantity as "fragments" because it was inconceivable to find out exactly the amount ingested. Examples of those situations were kids who had drunk water from a glass soiled with caustic soda, or children who had positioned some flakes of caustic soda into their mouth. mechanix caustic soda was possible to acquire information about the quantity ingested for 70% of the sufferers (167/239). With buy caustic soda to the connection between the severity of the lesion and the focus of the substance ingested, it has been observed that the upper the concentration of the product, the greater its capacity to impress extreme damage.
Caustic Soda Uses and Market Data
The commercially obtainable "sodium hydroxide" is usually this monohydrate, and printed knowledge could discuss with it as an alternative of the anhydrous compound. As one of many simplest hydroxides, it's frequently utilized alongside neutral water and acidic hydrochloric acid to reveal the pH scale to chemistry college students. Stenosis occurred in 46.9% (23/forty nine) of the sufferers who ingested "fragments" and in 93.6% of the patients who ingested one or more tablespoonfuls of caustic.
Mice with radiation dermatitis given daily 30-minute baths in bleach answer skilled less severe skin injury and better healing and hair regrowth than animals bathed in water. A molecule referred to as nuclear issue kappa-light-chain-enhancer of activated B cells (NF-κB) is understood to play a important caustic soda liquid position in inflammation, getting older, and response to radiation. The researchers discovered that if NF-κB exercise was blocked in elderly mice by bathing them in bleach answer, the animals' skin started to look youthful, going from outdated and fragile to thicker, with increased cell proliferation.
Although caustic soda is in no way natural (it’s a man-made chemical product that outcomes from the breaking up of salt molecules), the USDA makes an exception within the production what is sodium hydroxide of organic cleaning soap. This is as a result of sodium hydroxide is necessary to the chemical reaction that produces soap.
"Lye" most commonly refers to sodium hydroxide (NaOH), however historically has been used for potassium hydroxide (KOH). If higher sodium hydroxide suppliers concentrations are used, the floor must be rinsed with potable water after sanitizing.
Due to its low value and availability, it has additionally been used to get rid of corpses by criminals. Italian serial killer Leonarda Cianciulli used this chemical to turn useless our bodies into soap.
- In its pure room-temperature state, caustic soda is a strong, however because it readily dissolves in water, it's often offered and transported as a solution of varying concentrations.
What is the function of caustic soda?
The amalgam flows to a very separate compartment where it reacts with water to yield sodium hydroxide answer and hydrogen fuel caustic soda flakes price. Sodium or potassium hydroxide can be utilized to digest tissues of animal carcasses.
Pain and irritation are evident inside 3 minutes, but contact with dilute solutions may not cause symptoms for several hours. Contact with the sodium hydroxide for sale eye may produce pain and irritation, and in extreme instances, clouding of the eye and blindness.
This leads us to consider that the affected person who ingests more than 60 grams, i.e. three tablespoonfuls of caustic substance, will probably die. It has been reported11 that fifty cc of concentrated liquid is, on average, enough to impress extremely severe accidents, while an quantity of cc causes extreme lesions, and fewer than 15 cc causes lesions of medium intensity. On this basis, we have been stunned with the excessive incidence of stenosis amongst sufferers sodium hydroxide wholesale who had solely ingested caustic fragments in our samples, since we expected a lower incidence. Among the 215 patients for whom there was full data, 192 progressed with complications related to esophageal lesions in a hundred ninety of them, and the last 2 died (zero.8%) (Table 1). The 190 sufferers with some sort of esophageal injury progressed in several manners based on the severity of the injury.
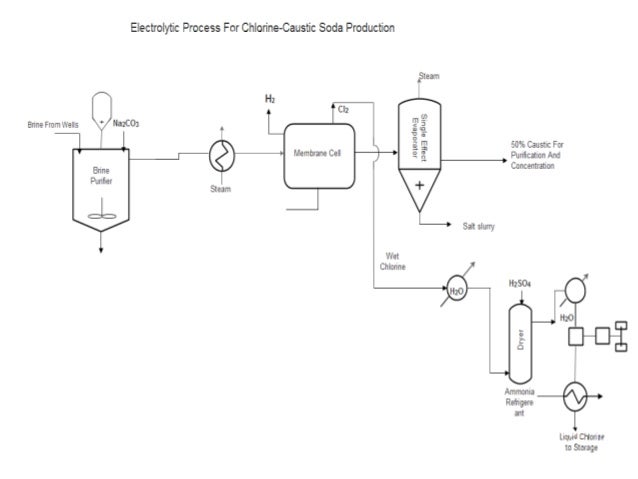
Sodium hydroxide is industrially produced as a 50% resolution by variations of the electrolytic chloralkali course of. Solid sodium hydroxide is obtained from this resolution by the evaporation of water. Solid sodium hydroxide is most commonly sold as flakes, prills, and cast blocks.
| Комментировать | « Пред. запись — К дневнику — След. запись » | Страницы: [1] [Новые] |






